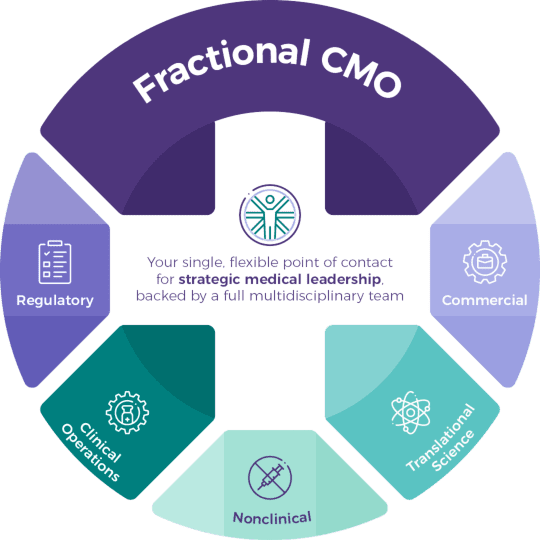
Unlike traditional CROs or standalone consultants, tranScrip combines strategic CMO-level oversight with the full depth of its multidisciplinary teams in regulatory, nonclinical, CMC, clinical operational, drug safety, commercial and translational science.
This means companies don’t just get a single expert, but a connected bespoke network – as needed – that can anticipate risks, shape development strategy, and execute solutions seamlessly. The result is flexible, informed leadership, faster decision-making, and the assurance that every critical step is guided by people who have done it before.